WHO recommends second malaria vaccine for children, rollout early next year.

The R21/Matrix-M vaccine, manufactured by Serum Institute of India, is already approved for use in Burkina Faso, Ghana and Nigeria.
The World Health Organization (WHO) has recommended a second malaria vaccine, a decision that could offer countries a cheaper and a more readily available option than the world’s first shot against the parasitic disease.

The R21/Matrix-M, developed by Britain’s Oxford University, can be used to curb the life-threatening disease spread to humans by some mosquitoes, the WHO said on Monday.
WHO Director-General Tedros Adhanom Ghebreyesus said the UN health agency was approving the new malaria vaccine based on the advice of two expert groups, recommending its use in children at risk of the disease.
“As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two,” Tedros said.
The R21/Matrix-M vaccine is manufactured by the Serum Institute of India and has already been approved for use in Burkina Faso, Ghana and Nigeria.
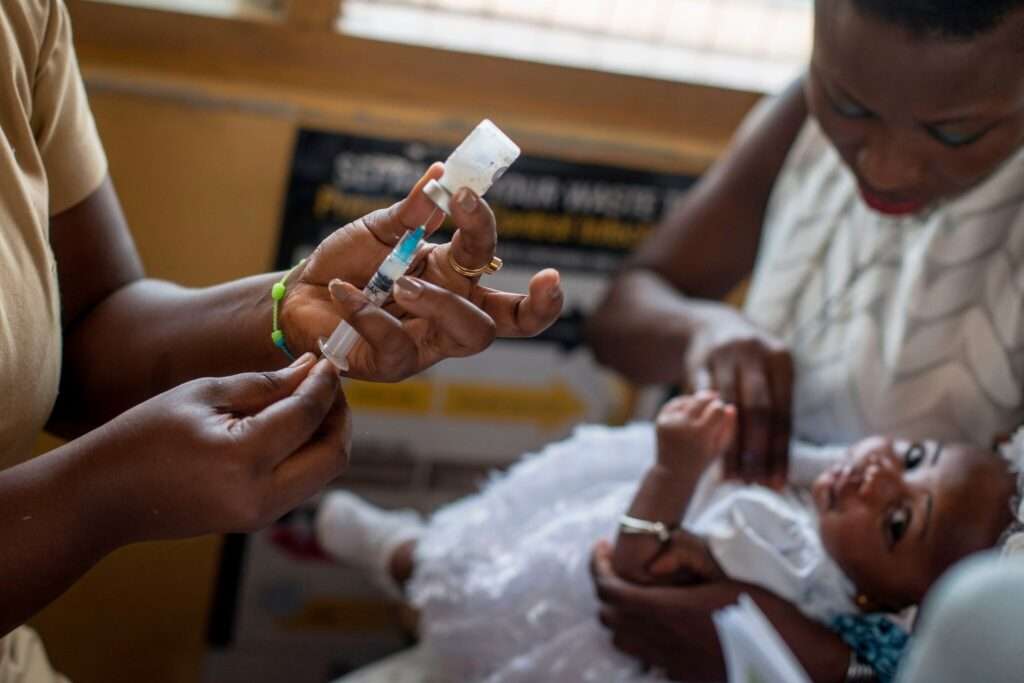
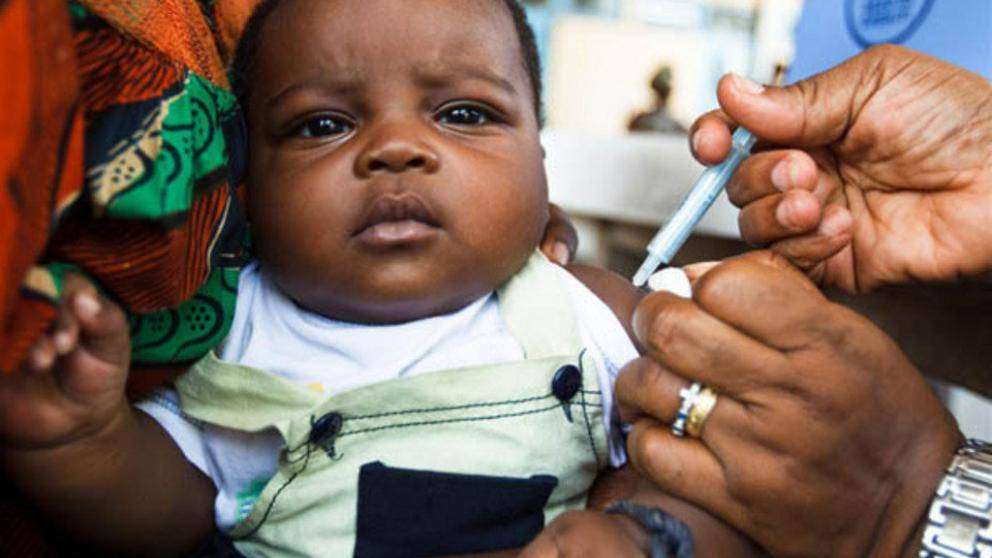
The vaccine will be rolled out in those African countries in early 2024 and will be available in mid-2024 in other countries, Tedros said, adding that doses would cost from $2 to $4.
“WHO is now reviewing the vaccine for prequalification, which is WHO stamp of approval, and will enable GAVI [a global vaccine alliance] and UNICEF to buy the vaccine from manufacturers,” Tedros said.
Oxford University developed the new three-dose vaccine with help from the Serum Institute of India. Research has suggested it is more than 75 percent effective and protection is maintained for at least another year with a booster.
Neither of the malaria vaccines available, however, stop transmission, so immunisation campaigns alone won’t be enough to stop epidemics. Efforts to curb the disease are also being complicated by increasing reports of resistance to the main drugs used to treat malaria and the spread of invasive mosquito species.
Takeda Pharmaceuticals’ vaccine against dengue for children aged six to 16 living in areas where the infection is a significant public health problem.
Dengue, common in tropical and subtropical climates, is a viral infection spread from mosquitoes to people.
Takeda’s vaccine, called Qdenga, was shown in trials to be effective against all four serotypes of the virus in people who were previously infected with dengue, Hanna Nohynek, chairwoman of the WHO’s Strategic Advisory Group of Experts on Immunization, told journalists.
She added, however, that there remained uncertainty about its performance against serotypes 3 and 4 in people who have not been infected previously.
Nearly 1,000 people have died of dengue this year in an ongoing epidemic in Bangladesh, the country’s worst outbreak of the disease.
The WHO’s strategic advisory group also recommended a simplified single dose regime for primary immunisation for most COVID-19 vaccines to improve acceptance of the shots at a time when most people have had at least one prior infection
Source: Aljazeera
Richard Koomson| mediacentralonline.info |Ghana
kindly send us your stories on our WhatsApp line 0500004727






